Clinical Trials
Vaccine clinical trials, a type of clinical research study, are medical studies that involve people. This is a process of evaluating the safety, immunogenicity, and protective efficacy in humans of a novel vaccine candidate before it can be licensed for use.
Clinical trials are carefully designed, reviewed, and completed, and need to be approved by the Food and Drug Administration (FDA) before they can start. People who meet the inclusion criteria can take part in clinical trials.
Vaccine development is a lengthy and a rigorous process.
Before a vaccine candidate is even tested among people, it has to pass the preclinical stage of development first where it is tested on animals to see if it will cause an immune response.
PH Response
The clinical development is divided into three phases:
For phase 1, the vaccine is tested in small groups of people to assess its safety. The coverage is expanded in Phase 2 to assess the efficacy, right dosage, and ensure that the desired effects are achieved. Phase 3 covers a larger group of people to confirm its efficacy and safety when compared to other treatments.
This whole process usually takes years to complete.
In the Philippines, our clinical trials are categorized into two:
WHO Solidarity Vaccine Trial, as approved by the Inter-Agency Task Force for the Management of Emerging Infectious Diseases Resolutions (IATF) Resolution No. 47, as part of our country’s commitment to make the COVID-19 vaccine available in the soonest time possible for the Filipino people.
- The Solidarity Vaccine Trial is a global action led by WHO to:
- to evaluate vaccine candidates as quickly as possible (identify vaccine candidates and their progress)
- to define the desired characteristics of safe and effective vaccines to combat the pandemic, and
- to coordinate the clinical trials across the world giving the best chance of safe and effective vaccines for all
Independent Trials, where other vaccine developers can conduct independent clinical trials in the Philippines provided that they will be able to fund their own trials and have their application registered and approved by the FDA.
The Task Group on Vaccine Evaluation and Selection (TG VES), composed of the Department of Science and Technology, Department of Health, Food and Drug Administration, Research Institute for Tropical Medicine , Department of Foreign Affairs, Department of Trade and Industry – Board of Investments and the National Development COmpany, oversees the applications for the conduct of clinical trials in the country, and evaluate the results of these trials. The TG VES provides recommendations on which vaccines will be considered for negotiation and procurement by evaluating: safety, efficacy and other relevant data from clinical trials, existing evidence and updates on vaccine development, and international recommendations on vaccine use.
Why should the Philippines participate in the ongoing clinical trials for COVID-19 vaccines?
Considering the current limitations of our country in terms of vaccine development, participation in clinical trials of the most advanced candidates developed by international partners will be the best short-term strategy for us. Participation in these clinical trials will secure a shorter and easier route in registration with the Philippine Food and Drugs Administration (FDA).
By pursuing collaborations and by participating in clinical trials, we are hoping to:
- To provide efficacy and safety data for a vaccine that is directly attributed to the Filipino population
- To generate necessary long-term data on safety and efficacy of vaccines, which is also necessary for CPR application even for vaccine candidates with an EUA
- There are still a lot of unknowns about COVID-19 especially on whether this will be like flu in the long run which will need follow-up yearly vaccinations.
- With new COVID-19 variants being identified, tweaking of vaccine candidates or their dosing schedule may also be needed to improve their efficacy and clinical studies are needed to confirm this.
Basics on CT
-
LOVE FOR SELF AND FAMILY
Giving your “Yes” is crucial in our rapid search for COVID-19 vaccine. The information generated from your participation is valuable in order for the research to move forward. Successful or not the information is helpful in showing what didn’t work and pushing research in a different direction.
-
LOVE FOR COUNTRY
When you participate in the trial, you are helping our country to have access to potential vaccine candidates.
-
SOLIDARITY WITH THE WORLD
By joining the trial, we help in the rapid worldwide search for COVID-19 vaccine. You are becoming part of the solution to end the pandemic.
Benefits
What benefits will we get from participating in vaccine clinical trials?
Clinical trials are important for discovering new treatments for diseases, as well as new ways to detect, diagnose, and reduce the chance of developing the disease. They also show researchers what does and doesn’t work in humans that cannot be learned in the laboratory or in animals.
Here are the benefits that we can get from participating in vaccine clinical trials:
- We may get a new vaccine for a disease before it is available to everyone.
- We will have the chance to help others get a better vaccine for existing health problems in the country.
- Volunteers of clinical trials are vital to the process of improving medical care. Many people volunteer because they want to help others.
Risks
What are the risks to consider?
Clinical trials are ongoing research, which means that we are not sure of the outcomes and that they may come with risks:
- Clinical trials are experimental, so there is always a possibility that the vaccine may not work.
- There is a possibility that the new vaccine may cause some side effects and in extreme cases, adverse effects.
- You may NOT be part of the treatment group that gets the new treatment. Inste
Emergency Use Authorization is not the same with an approval for product registration.
| Clinical Trial | Emergency Use Authorization | Product Registration |
|---|---|---|
| New vaccine is being tested on people to assess if it is safe and efficacious. In this stage, vaccine candidates are tested for their safety and efficacy. Further, clinical trials are NOT vaccination programs. | Unapproved vaccine is authorized for limited use as it is deemed safe and efficacious based on review of interim data of Phase III clinical trials. | New and approved vaccine is proven safe and efficacious based on review of complete data from Phase III clinical trials and can therefore be purchased and used for vaccination programs. |
Frequently Asked Questions - Safety and Efficacy of Vaccine in CT
All Vaccines undergo clinical trials before they are introduced. Under the Bayanihan Act II, we have allowed the entry of vaccines which have undergone Clinical Trial Phase III (normally up to Clinical Trial Phase IV). In the published results of clinical trials conducted for COVID-19 vaccines, a lower risk of symptomatic COVID-19 was observed with vaccination compared to placebo and the incidence of serious adverse events was low.
An example is the most recently approved vaccine for emergency use authorization (EUA) by the US FDA, the BNT162b2 mRNA COVID-19 vaccine (Pfizer and Moderna) which was characterised by short term, mild-to-moderate pain at the injection site, fatigue, and headache. However, there were reported deaths but none of these was considered by the investigators to be related to the vaccine or placebo, and no COVID-19 associated deaths were observed.
Vaccines shortlisted for evaluation were scored and weighted following the WHO Criteria for COVID-19 Vaccine Prioritization and WHO Target Product Profiles for COVID-19 Vaccines with additional criteria and considerations suited for local needs.
The following are the criteria that were used by the Task Group:
1.Potential Efficacy and Safety based on Published Phase III Interim Result and/or with EUA
2.Technology Platform (Reliability and Stability Related to Storage Requirement)
3.Safety based on Phase 1 and 2 clinical trials
4.Immunogenicity (Potential efficacy based on Phase II CT )
5.Vaccine Implementation (i.e Dosing Schedule)
6.Track record of company in developing and/or manufacturing other vaccines
For the WHO Solidarity Vaccine Trials, part of the protocol of the trial is the monitoring of the volunteers itself. All activities will be undertaken by clinical experts from various institutions and team of trialists with Good Clinical Practice (GCP) training. Active surveillance will be done wherein weekly phone calls from the study team to monitor for COVID symptoms and potential adverse events. Follow-up monitoring will be done six (6) months up to 1.5 years after the last vaccination has been done. Specifically, monitoring will be as follows:
- Case monitoring: Will be conducted for each individual from 14 days after the final vaccination and the cases collected 14 days after the final vaccination will be considered as valid cases.
- Efficacy follow-up will include weekly contacts to reduce loss of trial participants and to increase likelihood of detecting COVID-19 disease. Blinded study follow-up, including for adverse events, will be planned for at least one year, although for vaccines found to be substantially protective, vaccine and placebo recipients may be unblinded sooner.
- Different vaccines may possibly have different effects on different races. Hence, the conduct of local clinical trials in the Philippines will provide the vaccine developers efficacy and safety data that is directly attributed to the Filipino population.
- An EUA allows only limited use of vaccines for a certain priority group or sector. Vaccines under EUA cannot be used yet for mass vaccinations or for populations not covered by the current trials (e.g. pregnant women, those below 18 years of age, etc.).
- Long-term follow up data on safety and efficacy which can be achieved by continuing Phase III clinical trials for at least another 12 months even while there is already an EUA are still necessary to generate the evidence needed for full market authorization.
- Targeted trials on specific groups will still need to be done as well to be able to provide basis on the use of a vaccine on such groups or a specific population.
- Considering that most vaccines being procured under EUA right now have not been studied in Filipinos, local trials can generate the necessary long-term data on safety and efficacy of vaccines.
It is too early to know if COVID-19 vaccines will provide long-term protection. Additional research is needed for us to determine the long-term effect of the COVID-19 vaccines. However, it’s encouraging that available data suggest that most people who recover from COVID-19 develop an immune response that provides at least some period of protection against reinfection – although we’re still learning how strong this protection is, and how long it lasts.
The holder of the EUA shall have a comprehensive pharmacovigilance system for their product following the system or protocol for a registered drug and biological product. They shall ensure compliance to the Risk Management Plan (RMP), which contains information on product safety profile and explains the measures to characterize the risk including ongoing, new studies or additional activities. The summary of the RMP shall be published in the FDA website. Any deviation from or changes to the manufacture and changes in label of the product must be notified with the FDA. The pharmacovigilance obligations and post-authorization commitments of the holder of the EUA shall be shared by the national procurer and health program implementers to the fullest extent possible and applicable.
The following are some of the other vaccines developed and/or commercialized by some of the vaccine developers with a COVID-19 vaccine in advance stage of development:
- Pfizer: Pneumococcal (conjugate) Prevenar 13; 10 others vaccines in the pipeline
- Moderna: Cancer and Prophylactic vaccines (both mRNA)
- AstraZeneca: FluMist Quadrivalent flu vaccine
- Gamaleya: Ebola
- Janssen: Vaccines for bacterial infections
- Novavax: Seasonal Influenza, RSV, Ebola, MERS, SARS
- Clover: In the pipeline: HIV, RSV, Influenza
- Anhui: 17 clinical trials were registered on vaccines for PTB, BCG, Norovirus
- Sinovac: Healive (Hep A), Bilive (Combined Hep A and Hep B), Anflu (Seasonal Influenza), Panflu (Pandemic Influenza H5N1), and Panflu.1 (Pandemic Influenza A H1N1)
- Bharat Biotech: 14 vaccines including for Hib, polio, A(H1N1), rabies, rotavirus, typhoid
- Sinopharm: No info found for other vaccines but they have therapeutic products and medical devices and equipment
Clinical trials have several stages:
Phase 1: The candidate vaccine is given to a small number (10-100) of healthy adults with the primary aim of assessing safety
Phase 2: If the the candidates vaccine is found safe in Phase 1, it will be given to 100-1000 participants to determine how effectively it stimulates immune response and if there are any side effects;
Phase 3: If the vaccine is found to be safe and effective in Phase 2, it will be given to thousands of people (as much as ~30K) to determine if it protects a large population from the disease and whether there are any uncommon or serious side effects.
Even for vaccines that will eventually be given approval, monitoring of people who are vaccinated will still be done as there are very rare (i.e. 1 in 100,000 or 1 in 500,000 people) side effects that large clinical trials may not capture, or the clinical trials may not have included groups who may have higher risk of side effects than those who participated in the trials (e.g. pregnant women, older population
Vaccines are some of the most rigorously tested medical products today, and the COVID-19 vaccines are no different. The fact that the COVID-19 vaccines are available for use less than a year since the virus was discovered was not because corners were cut in the conduct of the clinical trials. Instead, the technology used to make the vaccines are the result of years of basic science research and experience with other outbreaks, as well as the continuous advancement in science and technology. All the vaccines which underwent Phase 3 clinical trials have undergone rigorous evaluation and examination by independent safety monitoring boards and have been scrutinized by the strictest regulatory agencies in the world. The vaccines with published Phase 3 trials show remarkable efficacy and impressive safety profiles. Most side effects are mild and self-limiting and very rare reports of severe adverse events. It must be noted that the results of these Phase 3 trials are “interim” because these safety and efficacy data are short term. The Phase 3 studies will continue on to completion in 1-2 years and it’s only then when we will have the full picture of its long term capability to provide protection. Having said this, in the setting of a raging pandemic where people at highest risk for contracting the disease must be protected immediately, the interim Phase 3 results and the resulting Emergency Use Authorizations from the various regulatory agencies worldwide are reliable markers that should rebalance the risk-benefit assessment towards receiving the vaccines.
Safety monitoring. The sponsor or the local CRO will be responsible for monitoring the progress of independent clinical trials. They are required to submit a monthly study progress report to the FDA. On top of that, FDA has an established process for reporting suspected adverse reactions, FDA AO No. 2016-026 which details the timelines and actions to be taken should an adverse event occur.
WHO SVT
| WHO SVT | Independent trials |
|---|---|
| The Philippines as approved by the IATF Resolution No. 47 will participate in the WHO Solidarity Vaccine Trials as part of the country’s global commitments to collective and inclusive efforts on COVID-19 vaccine development. | Other vaccine developers can conduct independent clinical trials in the Philippines provided that they will be able to fund their own trials and have their application registered and approved by the FDA. |
| Implemented by the World Health Organization, funded by the Philippine government | Implemented and funded by independent private companies and their respective CROs |
| Evaluates safety and efficacy of “several preventive candidate SARS-CoV-2 vaccines” under development | Evaluates safety and efficacy of one candidate vaccine for each independent private company |
| Adaptive, rapid, community-based trials | Hospital/Trials site-based recruitment |
| Large, international, multi-site,
individually randomized controlled clinical trial |
Study design varies for every independent private company |
| WHO SVT shall be prioritized in the assignment of Trial Zones, as stated in the Zoning Guidelines approved by the IATF | Separate trial zones will be assigned to independent trials, such that they are equally and rationally distributed to avoid competition in subject recruitment. |
| An independent Data and Safety Monitoring Committee (DSMC) will be formed by TG on Evaluation and Selection to monitor safety of vaccine candidates under WHO SVT while the study is ongoing. | The sponsor or local CRO will monitor the progress of clinical trials. However, they are required to submit a monthly study progress report to the FDA. |
| The WHO SVT team will oversee the recruitment, in partnership with local government units in designated trial sites. | Recruitment will be done through the local CROs which should follow the IATF-approved zoning guidelines |
As we gear for the conduct of these clinical trials, we are also counting on the support of our local chief executives to generate successful outcomes.
To summarize the crucial role of our local chief executives, we would like to share with you this acronym, Lingkod TAPAT:
- Tumulong sa pangangasiwa ng clinical trials kasama ang SVT team (Assist in facilitating the clinical trials in partnership with the WHO SVT team)
- Alamin at ipagbigay alam sa WHO SVT Team ang 3 klase ng lugar na kailangan para sa trial: may mataas na kaso ng COVID-19, may mga potensyal na boluntaryo sa trial, may pasilidad sa pangangasiwa ng trial (Identify 3 key locations for the clinical trial – places with high number of COVID-19 cases, places where we can recruit volunteers, places with the facility to conduct the clinical trial)
- Pamunuan ang preparasyon sa pamayanan para sa clinical trials (Lead the preparations needed in the selected trial sites)
- Asikasuhin ang pagbuo ng Community Advisory Board (CAB) (Form the Community Advisory Board)
- Transportasyon ng mga kalahok (Facilitate and arrange the transportation needed for appointments, follow-ups, etc.)
- Assisting in conduct of Vaccine Trial in various sites identified
- Educating respective barangays on the role of clinical trials in vaccine development and safeguards in place (i.e. informed consent process, management of potential adverse events)
- Supporting info dissemination on difference of vaccine clinical trials and regular immunization programs
Independent Clinical Trials
For independent clinical trials, discussions are ongoing with 25 biotech and pharmaceutical companies from different countries. To date, approved independent clinical trials will be conducted by:
- Sinovac
- Janssen Pharmaceuticals
- Clover Biopharmaceuticals
- WestVac Biopharma Co., Ltd.
- Inovio Pharmaceuticals, Inc.
- Shenzhen Kangtai Biological Products Co., Ltd. and Beijing Minhai Biotechnology Co., Ltd.
- Livzon Mabpharm Inc.
These clinical trials will be handled by private CROs, and will follow the protocols of the FDA. Every application undergoes a rigorous review process by the FDA, SJREB and partner evaluators, before they are approved.
You may access the approved clinical-trial applications uploaded on the Philippine Health Research Registry.
Glossary of CT terms
- Adverse reaction – An unwanted effect caused by the administration of drugs
- Clinical trial – A research study involving humans to determine whether new drugs or treatments or vaccines are both safe and effective
- Control group – The standard by which experimental observations are evaluated. In many clinical trials one group of patients will be given an experimental drug or treatment, while the control group is given either a standard treatment for the illness or a placebo
- Data safety and monitoring Committee (DSMC) – An independent committee composed of community representatives and clinical research experts that review data while a clinical trial is in progress to ensure that participants are not exposed to undue risk.
- Dosage – The amount of drug administered to a patient or test subject over the course of the clinical study
- Effectiveness The extent to which a specific intervention, when used under ordinary circumstances, does what it is intended to do.
- Efficacy The extent to which an intervention produces a beneficial result under ideal conditions.
- Eligibility criteria – The medical or social standards determining whether a person may or may not be allowed to enter a clinical trial
- Good clinical practice (GCP) – The rules for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials. GCP provide assurance that data and results are based on sound scientific and ethical research
- Informed consent – The process of learning the key facts about a clinical trial before deciding whether to participate. It is also a continuing process throughout the study to provide information for participants.
- Institutional review board (IRB) A committee of physicians, statisticians, researchers, community advocates, and others that ensures that a clinical trial is ethical and that the rights of study participants are protected
- Interim analysis – Analysis comparing intervention groups at any time before the formal completion of a trial, usually before recruitment is complete.
- Investigational new vaccine – A new vaccine that is used in a clinical investigation
- Pharmacovigilance – The science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem
- Placebo – An inactive pill, liquid, or powder that has no treatment value. In clinical trials, experimental treatments are often compared with placebos to assess the treatment ’ s effectiveness.
- Placebo effect – A physical or emotional change, occurring after a substance is taken or administered, that is not the result of any special property of the substance
- Preclinical – The testing of experimental therapies in the test tube or in animals — the testing that occurs before trials in humans may be carried out.
- Protocol – A study plan on which all clinical trials are based. The plan is carefully designed to safeguard the health of the participants as well as answer specific research questions.
- Randomization – A method based on chance by which study participants are assigned to a treatment group.
- Side effects – Any undesired actions or effects of a drug or treatment. Experimental drugs must be evaluated for both immediate and long-term side effects.
IEC Materials - Explainer Videos
IEC Materials - Primer
Good Clinical Practice for Community Health Workers
DISCLAIMER
This GCP training course is intended for community health workers (CHWs) in the Philippines participating in Covid vaccine trials. The DOST and DOH certify that a CHW has met the minimum requirements for Basic Good Clinical Practice during community engagement. Moreover, the certificate may be used as evidence of updated GCP training in the conduct of vaccine community trials. But this short term training does not meet the initial training requirements for clinical investigators who need longer training hours to conduct research.
PATNUBAY SA MGA KALAHOK NG GOOD CLINICAL PRACTICE FLIPPED CLASSROOM
Bilang paghahanda sa COVID-19 vaccine clinical trials sa Pilipinas, naglunsad ng Basic Good Clinical Practice (GCP) Training para sa Community Health Workers ang Task Group on Vaccine Evaluation and Selection, na pinamunuan ng Department of Science and Technology (DOST) kasama ang Department of Health (DOH).
Ang pamamaraan ng pagtuturo na gagamitin sa training na ito ay “flipped classroom” kung saan ang mga CHWs ay maaaring mag-aral ng mga nakatalagang paksa sa bahay batay sa kanilang sariling oras at bilis. Ang pamamaraang ito ay nakikitang mabisa sa mga mag-aaral na may limitadong oras upang dumalo sa mahabang sesyon o panayam.
Layon ng online training na bigyan ang CHWs ng tamang kaalaman sa kung paano pangangalagaan ang karapatan ng mga kalahok sa clinical trials. Ang aktibidad na ito ay siya ring magsisilbing pagkakataon upang tugunan ang mga tanong ng CHWs tungkol sa clinical trials.
Ang Basic GCP Training na ito ay libre para sa Community Health Workers.
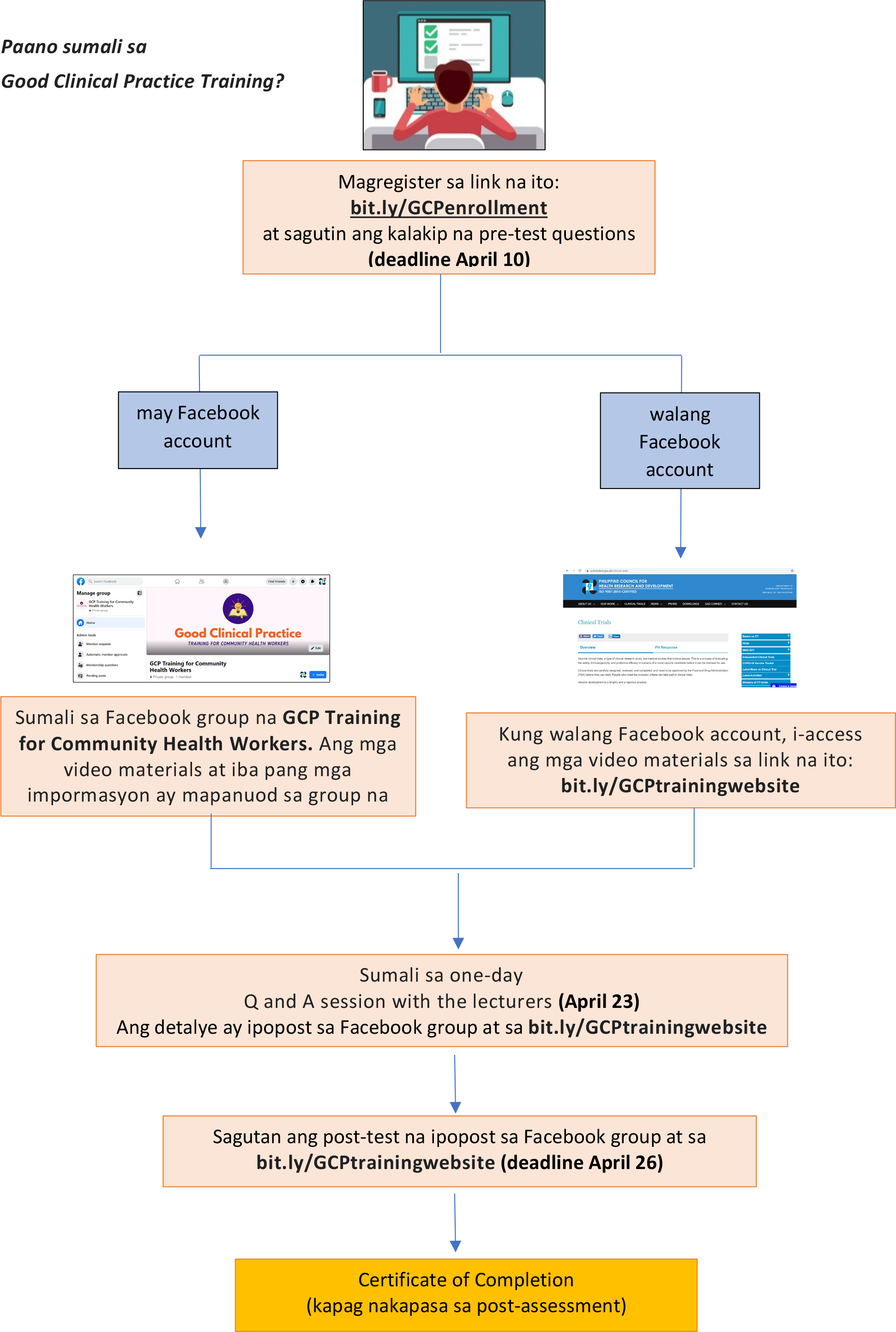
Sino ang maaaring sumali sa online GCP Training?
Ang lahat ng Community Health Workers gaya ng City Health Worker, Municipal Health Worker, Barangay health worker, mga lisensiyadong doctor, nurse, midwife at pharmacists na nagsisilbi sa mga pampublikong health centers at ospital, at ang iba pang lisensiyadong health professionals na nagtatrabaho sa mga pang pribadong klinik o ospital ay maaaring sumali sa online GCP Training.
Ano ang kailangan para sa pagsali?
- Kailangan ng gadget gaya ng laptop, mobile phone, tablet na may internet connection at kayang manuod ng video gamit ang YouTube at mag-access ng website
- Kailangang may email account
- Facebook account (optional)
MGA ABISO:
| Aktibidad | Petsa |
|---|---|
| Registration/ Enrollment
Pagsagot ng Pre-Test |
Marso 29 – Abril 10, 2021 |
| Panunuod ng video lessons | Abril 10 – Abril 23, 2021 |
| Paglahok sa Q and A Session with lecturers | Abril 23, 2021 |
| Pagsagot ng Post Test | Abril 26, 2021 |
Video Lessons
MGA ARALIN PARA SA GOOD CLINICAL PRACTICE FLIPPED CLASSROOM
PAALALA: Ang lahat ng materyal sa website, Youtube page at Facebook group ng DOST-PCHRD para sa Good Clinical Practice Training ay protektado ng copyright sa ilalim ng Copyright Act. Hindi maaaring kopyahin, ipamahagi, i-publish, ipakita, baguhin, lumikha ng mga gawaing hango, magpadala, ibenta, o sa anumang paraan ay pagsamantalahan ang anumang naturang nilalaman. Ang pagkopya o pag-iimbak ng anumang nilalaman ay malinaw na ipinagbabawal nang walang paunang pahintulot ng DOST-PCHRD at ng may-ari ng copyright. Para sa pahintulot na magamit ang mga lectures, mangyaring makipag-ugnay sa ricud-comm@pchrd.dost.gov.ph.
Ang nilalaman ng Facebook group at website ay ibinibigay lamang para sa mga hangaring pang-edukasyon at impormasyon. Ang PCHRD ay maaaring magdagdag, mag-ayos o magtanggal ng anumang nilalaman, patakaran, pamamaraan o regulasyon ng GCP Training.
Sa araling ito ipapaliwanag ang mga pangunahing konsepto tungkol sa clinical trials at Emergency Use Authorization (EUA) ng mga bakuna para sa COVID-19. Ipapaliwanag din kung bakit mahalaga ang paglahok ng Pilipinas sa clinical trial at ang dalawang klase ng clinical trial na isasagawa sa bansa.
Pagkatapos ng pag-aaral, dapat matutunan ng kalahok ang mga sumusunod:
- Ano ang clinical trials and Emergency Use Authorization(EUA)?
- Ano ang tungkulin ng Task Group on Vaccine Evaluation and Selection (TG VES)?
- Ano ang pagkakaiba ng Clinical Trials at EUA?
Lecturer:
Dr. Jaime C. Montoya
Executive Director
DOST-Philippine Council for Health Research and Development
Estimated duration:
20 minuto
Ipapaliwanag sa araling ito ang papel na ginagampanan ng mga Community Health Workers sa pagsasagawa ng clinical trials. Nilalayon nitong ipaunawa ang wastong mga kasanayan na dapat sundin ng mga CHWs upang matiyak na ang mga clinical trials ay sumunod sa mga ethical standards.
Pagkatapos ng pag-aaral, dapat matutunan ng kalahok ang mga sumusunod:
1. Ano ano ang mga dapat tandaan ng Community Health Workers sa pagsasagwa ng clinical trials?
2. Ano ano ang mga hindi dapat gawin sa pagsasagawa ng clinical trials?
3. Ano ang responsibilidad ng Community Health Workers sa pagsasagawa ng clinical trials?
Lecturer:
Dr. Cristina Torres
Coordinator
Forum for Ethical Review Committees in the Asia and Western Pacific Region
Estimated duration:
45 minutes
Ang araling ito ay magbibigay ng gabay sa kung ano ano ang mga dapat tandaan sa pag-rekrut ng mga kalahok at nagbibigay-diin sa kahalagahan ng pagkuha ng informed consent o pahintulot ng mga lalahok sa clinical trials na batay sa itinakdang etikal na pamantayan.
Pagkatapos ng pag-aaral, dapat matutunan ng kalahok ang mga sumusunod:
- Ano ang informed consent? Bakit ito mahalaga?
- Ano ano ang mga dapat tandaan sa pagkuha ng consent o pahintulot ng kalahok na pagsali sa clinical trials?
Lecturer:
Ms. Edlyn B. Jimenez
Coordinator
University of the Philippines Manila Research Ethics Board
Estimated duration:
22 minutes
Ang pagsubaybay sa mga clinical trials ay isang mahalagang hakbang sa pananaliksik. Sa araling ito, tuturuan ang mga CHWs kung ano ang kahalagahan ng diary card at paano ito ginagamit para sa safety reporting and documentation sa clinical trials.
Pagkatapos ng pag-aaral, dapat matutunan ng kalahok ang mga sumusunod:
- Ano ang Serious Adverse Events (SAE)?
- Ano ang diary card?
- Ano ang dapat malaman ng CHW sa paggamit ng the diary card?
- Bakit mahalaga ang diary card sa mga lalahok sa clinical trials?
Lecturer:
Mr. Al Ryan Baniqued
Country Head, Parexel Clinical Research Philippines Ltd. Corp
Estimated duration:
22 minutes
GCP Certificates Recipients
LIST OF GCP CERTIFICATE RECIPIENTS
The names listed here belong to trainees who have satisfied all the requirements of the GCP Training Program, which include the completion of: 1) enrollment 2) pre-assessment quiz, 3) attendance to the live Q&A session, and 4) post-assessment quiz, with a score of 60% and above. The DOST and DOH certify that the following community health workers have met the minimum requirements for Basic Good Clinical Practice during community engagement.
| 1. Abdul Javar Esturco | 242. Grace Macapagal | 483. Maricel Mendoza |
|---|---|---|
| 2. Abelaine Venida Tablizo | 243. Gracielle Ruth Adajar | 484. Marie Angelie So |
| 3. Adelene Celestra | 244. Gracieux Fernando | 485. marie cherry lynn fernando |
| 4. Adelyn A Duyag | 245. Hannah Lydia Bernardo | 486. Marie Eleonor T. Libunao |
| 5. Adjarael Joemal V. Malali, Jr. | 246. Hara Jenrose E. Caparro | 487. MARIE ELLA FLOR DE VERA URBANO |
| 6. Adrian G. Fernandico | 247. HARTZELL DASKEO | 488. Marie Florence R. Manaois |
| 7. Adrian John P. Bejarin, MD | 248. Hazel Floreta | 489. Marie Grace M.Omadto |
| 8. Adrian Segovia | 249. Hazel Lat | 490. Marie Lemyr Abelardo |
| 9. AEGAN MATTHEW V AMICAN | 250. Herbert Pascual | 491. Mariecor Tamayo |
| 10. Aemmanuelle Magno | 251. Heriberto Guballa | 492. Mariel Joy M. Atanacio-Lu |
| 11. Agnes Venessa Carungcong | 252. Humprey G. Icban | 493. Mariel Sevilla |
| 12. Aireen Patricia Madrid | 253. Ian Gabrielle Cuyno | 494. Marielle Eunice C. Ramajo |
| 13. Airene Biaco-Nacion | 254. Ian Nova Dela Camara | 495. Marieugenie Yazmin Pauline Daen |
| 14. Aldren R. Remon | 255. Ida Marie T. Lim | 496. MARIEZANE VITTO |
| 15. Aldrin Riel Boyano | 256. Imarzen Elepano | 497. Marilei Martinez |
| 16. Alejandro Ylaya | 257. Imelda Caole-Ang | 498. Marilou Madrid |
| 17. Alex V. Abelos | 258. Ira Fe Borillo | 499. Marilou Tan |
| 18. Alexander Berioso II | 259. Isagani Quidilla | 500. Mario Domingo |
| 19. Alexander Pring | 260. Isidro Sia | 501. Marisol Molina – Adorable |
| 20. Aljeane Ramillano | 261. IVY MARCEL VARIAS | 502. MARITESS OMBAO |
| 21. Almira A. Manzano | 262. Iza Donna R. Togbo | 503. Mark Clyde D. Daoana |
| 22. Almon Constantino | 263. Iza Mae Chamen | 504. Mark Gay-as |
| 23. Alsun Cabarles | 264. JACK BELARDO | 505. Mark Isaiah K. Co |
| 24. Alvel Tang-Manga | 265. Jaclyn Johnson-Magpantay | 506. Mark luigi ebreo |
| 25. Alvin C. Agustin | 266. Jacqueline Calaycay | 507. Mark Paolo Enrico U. Obias |
| 26. Alvin Chester P. Señir, MD. | 267. Jaffar Carag Pineda, M.D. | 508. Mark Pretzel Zumaraga |
| 27. Alvin Saed | 268. Jahzeel Acab | 509. Mark Rodrigo D. Mendros |
| 28. Alyssa Adeley Derramas | 269. Jake Anthony G. Albarico, RN, MD | 510. MARLON C. MALLILLIN, III |
| 29. Amapola Puaso | 270. Jamaica Marie M. Tumulak | 511. Marlou Del N. Tarranco |
| 30. Ana Aurelia Santos | 271. James Dominic Sayson | 512. Marlyn L. Vicerra |
| 31. ANA JESSA DE JESUS | 272. James Lloyd Galutera | 513. Marr Antoniette Boncan |
| 32. Ana Liza Duran | 273. Jamille Pasion | 514. Marri Jmelou Roldan |
| 33. Ana Pholyn Balahadia | 274. Janela Marie Co | 515. Marvic Sharmane R. Garcia-Tanael |
| 34. Ana Tanya Tullao | 275. Janette F. Pascual | 516. Marvin De Manuel |
| 35. Ana Tess Adis – De La Cruz | 276. JANICE D. DE LEON | 517. MARY ANGELICA V. LEGASPI |
| 36. Ana Trisha Okol | 277. Janice Regondola-Baltazar | 518. Mary Ann A. Wagas |
| 37. Andre Canaria | 278. Janine Aliza B. Birog | 519. Mary Ann Limbo |
| 38. Andrea Mendoza | 279. Jashca Angela M. Oquindo | 520. Mary Ann V. Leh |
| 39. Angel Ancheta Jr | 280. Jasmin S. Tamon | 521. Mary Davie Lynn L. Lo |
| 40. Angela Beatrice M. Martinez | 281. Jason James Lusabia | 522. Mary Grace Nicole Reyes |
| 41. Angelanna Ysais | 282. Jason Suquila | 523. MARY IODINE S. LACANIENTA |
| 42. Angeles T Alora | 283. Jason Syson | 524. Mary Jane Molina |
| 43. Angelica Fernandez | 284. Jean N. Guillasper | 525. Mary Joy D. Santos, PTRP |
| 44. Angelica Karina S. Ledesma | 285. Jeanelle Louise S. Dumalag | 526. Mary Joy G. Vasquez |
| 45. Angelica Marie T. Nario | 286. Jeannette Yee Ramiro | 527. Mary Luz Fiangaan |
| 46. ANGELIQUE AZUCENAS | 287. Jeciel Aropo | 528. Mary Lyn Pelingon |
| 47. Angelique Dominika Arellano | 288. JEFFREY LOBOS | 529. Mary Mildred D. Delos Santos |
| 48. ANGELITA RODRIGUEZ | 289. JEFFREY LUCERO | 530. Maximo B Axibal Jr |
| 49. Angelite weyne lahorra | 290. Jellian Razzele Kwok | 531. Melchor Gabriel |
| 50. Anna Karenina Causapin | 291. Jemimah Valentin | 532. Melissa Suzette Baesa |
| 51. Anna Marie Sandoval | 292. Jenelle Patricia D. Nival | 533. MERLINA L. CABRERA |
| 52. Annabel Aman | 293. Jennifer Javier | 534. Merlind Morales |
| 53. Annalyn Sugano | 294. Jennifer Rose Francisco | 535. MERLYN G. TAJAN |
| 54. Anne Dixie Lim | 295. Jennifer Therese Amorin | 536. Michael Calama-an |
| 55. Anne Mae Ros | 296. Jenny Lynn Vidanes | 537. Michael Dennis I. dela Paz, MD |
| 56. Anne Margaret Ang | 297. Jerome Senen | 538. Michael M. Resurreccion |
| 57. Anne Margaux Artates | 298. Jessa Louise Turreda | 539. Michelle Ann Miranda |
| 58. Antonio C Sison, MD | 299. Jesus R. Dabalos Jr | 540. Michelle Anne So Caamic |
| 59. Antonio C.Banario | 300. JEWELL NUÑEZ-SANGCATE | 541. Michelle Otayco |
| 60. April Anne Rivamonte Delola | 301. Jezel Samonte | 542. Michelle Sy |
| 61. Arianne Antonette O. Libed | 302. Jhon Mitchelle Wagas | 543. Mickhael Langit |
| 62. Aries O. Priagula | 303. Jian Carlo R. Narag | 544. Mignodel Morales |
| 63. ARJAY ABICHUELA | 304. JIAN JARA BUENA MANUEL | 545. Mikhaela Basila |
| 64. Arlene C. Parcasio | 305. JILLIAN AUDREY L. ANGELES | 546. Mila Sacamay |
| 65. Arlyn Cantillep | 306. Jireh Domingo Concepcion | 547. Milagros B. Rabe |
| 66. Armida Suller | 307. Jo Ann Margarette G. Tordil | 548. Milagros Jocson |
| 67. Armie Plando | 308. Joan Teodoro | 549. MILAGROS L. BORABO |
| 68. Arvin Alimurong | 309. Joaña Jeb Dungog | 550. Monica Bianca Hernandez Ordinario |
| 69. Ashley Ediamy D. Go | 310. Joana Sabong | 551. Monica Z. Imperial |
| 70. ATTY. ERIC G. CLAUDIO, Ph.D. | 311. Joann Joven | 552. Mylette Gan Delmas |
| 71. Aurea Mae Basa | 312. Joanna Mare A. Salinas | 553. Myrna De lara |
| 72. Aurora R. Valencia | 313. Joanne Charisse Q. Comayao | 554. Natasha F. Ilustrisimo |
| 73. Azenith Alegar-Barcia MD | 314. Joanne Marie Arcillas | 555. Nicole Mae Martinez |
| 74. Beatrice Alyssa Marie Sarte-Tan | 315. Jobellyn Catherine Reforma Dela Luna | 556. Nieves Capili |
| 75. Beatriz Barbara Kho | 316. Joey Kyle T. Mallari | 557. NOEL LAXAMANA |
| 76. Belinda Linayao | 317. Johanna Lise C. Silva | 558. Noel Nathaniel C. Napa |
| 77. Belle Ranile | 318. Johanne Fe C. Almonia | 559. NORIELL JONATHAN MAMACLAY |
| 78. Benedicto M Ancheta Jr | 319. JOHN CARLO LEE | 560. Norlia Macasalong |
| 79. Benita Gracia N. Viloria | 320. JOHN F. VILLEGAS | 561. OLIVE JOYCE ALABADO-LINDERO |
| 80. BERNARDO LABLABONG JR. | 321. John Lester C. Gonzaga | 562. Oliver Bryan Oliva |
| 81. Bianca Denise E. Edora | 322. John Lexter G. Mojica | 563. Omar Villalobos |
| 82. Bianca Nikkola Olonan | 323. John Louise D. Raval | 564. Orlando Filoteo |
| 83. Blessing J. Prado | 324. John Marie Idava | 565. ORPHA P.MONTILLANO,MD |
| 84. BRENAN IAN DC. CAPUNO | 325. John Mark Carabeo | 566. Paolo Pineda |
| 85. BRENDA CAMILLE JAVIER | 326. John Michael P. Tomagan | 567. Patria Pacquing |
| 86. Buena Dyanne Dawis | 327. John Oliver B. Villanueva | 568. Patricia Anne Concepcion |
| 87. Buford Boado | 328. John Patrick F. Ona | 569. Patricia Mae S. Santos |
| 88. CAILA NOELIN DE VILLA | 329. JOHN PAULO AUSTRIA | 570. Patrick Michael L. Roslyn |
| 89. CANDY ANGELICA M. SIGUA-CABADDU | 330. Joie Sheen Bastian | 571. Patrick Silagon Jr |
| 90. Carl Cabral | 331. Jomar Adams Ganding | 572. Paul John Bariuan |
| 91. Carla Jane Concepcion Magno | 332. Jomer Dennis B. Pinzon | 573. Pauline Joy Lorenzo |
| 92. Carla Mae C. Gialogo | 333. Jon Luzmar D. Empenado | 574. Paulo Maria N. Pagkatipunan |
| 93. Carlo Noel Santos | 334. Jonathan Disraeli Salvador | 575. Pearl Joy Cabarles |
| 94. Carlo Ordiz | 335. Jorell Victor S. Angeles | 576. Pedro Manzala |
| 95. Carlos T. Nunez, Jr. | 336. Jose Carlos Gana | 577. Phillip Aristotle R. Hermida |
| 96. Carmel Carabaña | 337. Jose L Tambuyat Jr | 578. Precious Emary Samonte |
| 97. Carmela D. Barcelona | 338. Jose Modesto III B. Abellera | 579. Prima Donna Napa-Lim |
| 98. Caryl Amisola | 339. Joselito Gardoce | 580. Qareem Pido |
| 99. Catherine Jennifer M. Catibog | 340. Joseph Adrian Buensalido | 581. Raamah Rosales |
| 100. Catherine May Hamtig | 341. JOSEPH ISRAEL R. GUANLAO | 582. Ramius Agustin Miguel D. Dizon |
| 101. Cattleya Marie Bragado | 342. Joseph Juico | 583. Ramoncito Yambao |
| 102. Cecilia Josefina Santos | 343. JOSEPHINE C. ABRAZALDO | 584. Randolf D. Cenon |
| 103. Cecille Alday | 344. Joshua Santos | 585. Randy Urtula |
| 104. Celegrace Zapanta, RN | 345. JOSIE P. DESNACIDO | 586. Ranier Ritchie Naldoza |
| 105. Cesaria Ang-Pasion | 346. Joy Abigail Hernandez | 587. Raphael Gabriel Tejada |
| 106. Charie Mae Daweg | 347. Joy Grace G Jerusalem | 588. Ray Dabon |
| 107. Charlene Santos-Bartolome | 348. Joy Palomares | 589. Ray Noel C Badal |
| 108. Charlie Labarda | 349. Joyce Parco | 590. Raymart Carl Amago |
| 109. Charlotte Anne Leung Chiw | 350. Joycel Fernandez | 591. RAYMOND EMMANUEL ALBA PINEDA |
| 110. Charmaine Bolusan | 351. Juan Karlo dela Cruz | 592. REGINA MARIE MARTIN |
| 111. Charmaine Micu-Oblefias | 352. Jude Tayaben | 593. Reinelle Ann B. De Leon |
| 112. Cherri Rachel Yarisantos | 353. Judith Charmaine E. Rosette, MD | 594. Remar Nell Z. Hapan |
| 113. CHERRY B. LAZATIN | 354. Judith G. Cabanela, MD | 595. Renand Jude Camarista |
| 114. Cherry Go-Palomar, M.D. | 355. Judy Carmela V. Rosario | 596. Renante C. Serrano Jr. |
| 115. Cherry Lou Guinto-Ilarde | 356. Julius Migriño, Jr. | 597. Renee Joy Neri |
| 116. Cheska Aira Yayen Abarra | 357. Jun Michael Bugarin | 598. Renelle Sebastian |
| 117. CHONA PATALEN | 358. Junhel Dalanon | 599. REYNALDO M. ALVAREZ JR |
| 118. Christian Bernard Cheng | 359. Justine Anne Sangil | 600. Reynen Rose S. Pausanos |
| 119. Christian Dominique S.C. YU | 360. Karen Bongas | 601. Rhealyn M. Ona |
| 120. Christian Ian Barrido | 361. Karina P. Lapinid | 602. Rhoda Lyn Arauag |
| 121. CHRISTIAN N. RAMOS, MD | 362. Karina Sze | 603. Rhodora Therese Torres |
| 122. Christianne Cabanos | 363. Karla Sophia Lumang | 604. Rhomina Jhona B. Suan, RN |
| 123. Christine Doncillo | 364. KATHERINE ANNE C. ACUIN | 605. Ria Carla C. Siccion |
| 124. Christine Grace Forcadas | 365. Katherine Rhea Cabantac | 606. Ria F. Valdez, M.D. |
| 125. Christine P. Manalili-Trinidad | 366. Kathleen Gonzales | 607. Ric Francis P. Miranda |
| 126. Ciara Margaret Nerona | 367. Kathryn Say | 608. Rica Ching |
| 127. Claire C. Ponce | 368. Katrina Bianca Chua | 609. Richard Abitong |
| 128. Clarissa A. De Guzman | 369. Katrina Concesa Refuerzo | 610. RICHELLIE PAGAR |
| 129. Clarissa Marie S. Tady | 370. Katrina Kamille C Fausto-Gonzales | 611. Richmae Gillera |
| 130. Coleen Jane Macariola | 371. Kayla Gabrielle C. Lecciones | 612. Rima Pabalan |
| 131. Colene Agatha Uy | 372. Kent Edbert Keh | 613. RINA JANE ALARCON |
| 132. Conrad Aron Valdez | 373. KEVIN CLARK T. LIM | 614. Rina Ong-Tanyang |
| 133. Crista Mae Fontanilla | 374. Kevin Paul B. Ferraris | 615. Rivero Opano |
| 134. Crystal Jmee G. Hernandez | 375. KHRISTINE JOY DE CASTRO MACAPAGAL | 616. Robelle Joan Peralta |
| 135. Cynthia Anzures | 376. Kiah Belle Felichi Songco | 617. Roberto M. Montaña |
| 136. Cynthia Nacpil | 377. KIMBERLY ANN PULGA | 618. ROBIN C. AT-AT |
| 137. CYREL ALYSSA LALIKAN | 378. KIMBERLY JEAN SURMION | 619. ROCHELLE REYES |
| 138. Cytesse C. Cambronero | 379. Kimberly Ruth Asilo | 620. Rochere Aubrey Olabe |
| 139. Dan Louie Renz Tating | 380. Kirsten Nicole Arcega | 621. Rocky Dizon |
| 140. Dan Micko F. Pedron | 381. Krissa Mae Bartilit | 622. ROD ERICK L. AGARRADO |
| 141. Daniel G. Salunga | 382. Krista Datuin | 623. Rodaliza Gumboc |
| 142. Daniel Patrick G. Mojica | 383. Kristeen Joy P. Mercado | 624. Rodderick Oclarino |
| 143. Danilo Baldemor | 384. Kristelie Mae Onda | 625. Rodelia V. Nicolas |
| 144. Danny Boy Gorospe | 385. Kristhine Abegail Gamiao | 626. Rodney Famero |
| 145. Darlene Sarah Isabel Pineda | 386. Kristian Alfonso Cueto | 627. Rodney Odesson Raguindin |
| 146. David Joseph Ong | 387. Kristin Catherine A. Balaaldia | 628. RODOLFO NATHANIEL ANGELES JR |
| 147. David Joshua Priela | 388. Kristine Bernadette Lawas | 629. Rodrigo Senador |
| 148. Dayang Sitti Shelna S. Corrales | 389. Kristine Joy Sapno | 630. ROI MARTIN B. PAJIMNA |
| 149. Denisse Mheryl S Bingcang | 390. Kristine Pascua Flores, MD | 631. Rojema A. Pangarungan |
| 150. Dennis Jose Sulit | 391. Kristle Aline C. De Roma | 632. Romeo C. Cruz Jr. |
| 151. Desiree Bautista | 392. Kriza Rosette A. Cadorna | 633. Romeo C. Tuazon Jr. |
| 152. Desiree Joy Pacana | 393. Krizchelle Ching Sai | 634. Romeo R. Mejia Jr |
| 153. Diana Peñaflor | 394. Krystle Marie Niñora | 635. Romerico Torres |
| 154. Diane Clarisse Garcia | 395. Lady Kristelle Van Z. Alaan | 636. Romiena Mae A. Santos |
| 155. Dianne Grace R. Calingasan | 396. Laura Andrea B. Rivera-Mapili | 637. Romulo Cunanan II |
| 156. Dianne Rose C. Mendoza | 397. Laurence Javier | 638. ROMULUS HILARIO |
| 157. Dimpna V. Bitagon | 398. Laurene Jeanne Ching | 639. Ronald Perez |
| 158. Dionisio Apdo Jr | 399. Lazo, Andrea T. | 640. Ronaldo Elepaño III |
| 159. DIVINA OCAMPO | 400. Lea Galia | 641. Roniefhel Apsay |
| 160. DOMINIQUE SEDANO | 401. Leedah Ranola-Nisperos | 642. Rosa Angela Dones-Delos Santos |
| 161. Donnie Saludes | 402. Lelyn R Mission | 643. Rosalie Remedios Ramirez |
| 162. Dr Erwin M. Faller | 403. Lemerie Anne P. Sunga | 644. Rosalyn Panao |
| 163. Dyan Kristel P. Sabalvaro | 404. LENARD CHARLES C. MONSADA | 645. Rose Christian H. Dayao |
| 164. Dyan Q. Sosing, MD | 405. Lestet Dimzon | 646. ROSE DYANE NUNAG |
| 165. Earl Adriane Cano | 406. Lexlee Joy M. Cabasal | 647. Roselle Poso |
| 166. Earl Francis Sumile | 407. Lhyka Pamintuan | 648. Roselle Sapanghila |
| 167. Earlan Bautista | 408. LIBRANDA | 649. Roselyn M. Panganiban |
| 168. Eden R. Go-Santos | 409. Liezl Malabanan | 650. Roselyn Maderse |
| 169. Editha P Jacer | 410. LILY ROSE DE LA CONCEPCION-CO | 651. Rotchelle Jane Collado |
| 170. Edmark C. Kamantigue | 411. Linelle Anne R. Aban | 652. Rowena A Raynera |
| 171. Edsel Martin | 412. Lodivica D Bandilla | 653. Rowena Escolar Chua |
| 172. EDWARD JEREMY JUANE | 413. Lopez, Arbee A. | 654. ROY CRESENCIO JR. LINAO |
| 173. Eileen Feliz Cortes-Garcia | 414. Lora Megumi Santiago | 655. Roy Luister Acos |
| 174. Elaine Marie Y. Omaña | 415. Lorenz Jacob Mangahas | 656. RUBIEANNE MALLILLIN |
| 175. Elaine Ooka | 416. Lorenza Legaspi | 657. Rubiliza Telan |
| 176. ELIJAH CULALA | 417. Lorraine H. Faeldon, M.D. | 658. RUSSELL ANNE MARIE CARANDANG, MD |
| 177. Elijah Mae C.Amistad | 418. Lotes Joy Sabuquia | 659. Ryan Andrew Manahan |
| 178. Elizabeth Go-Tan | 419. Louisiana Bianca Laurilla | 660. Ryan Cambaling |
| 179. Elizabeth K. Supelana | 420. Lovely Sanedrin | 661. Ryan de la rosa |
| 180. Ellicia Vern Mendoza | 421. Loyda Amor N. Cajucom | 662. Ryan James Suguitan |
| 181. Elmer Hilary Balbin | 422. Lozel Villadore | 663. Ryan Jes R. Reyta |
| 182. Elsa Dionisio | 423. Ma Elena Dalimbang | 664. Ryan Joseph C. Tuzon |
| 183. Emelita Padilla | 424. MA TERESITA DE GUIA-LIWANAG | 665. Ryan Parcon |
| 184. Em-Em Lamento | 425. Ma. Angelita Rabanal | 666. Salimah Langilao |
| 185. Emily Flores | 426. Ma. Barbara Monserrat Javier | 667. Sam Kevin C. Saclayan |
| 186. Emmanuel Ian Lorenz T. Pacao | 427. Ma. Carina L. Cruz | 668. Sarah Jane Toledo |
| 187. Ena Andrea M. Antonio | 428. Ma. Carina Rebueno | 669. Sean Paul C. Fiangaan |
| 188. Eric P. Cayabyab | 429. Ma. Elizabeth Fontanilla | 670. SHAHARA ALMAZORA DIANELA |
| 189. Erica Karla Dela Cruz | 430. Ma. Georgina H. Manzano | 671. SHANELLE MONLOY |
| 190. Erwin Palisoc | 431. Ma. Geraldine Ramos | 672. Shanelle Tan |
| 191. ESMERALDA ABLING | 432. MA. GERALDINE TONGCO | 673. Sharlene Marie Lao |
| 192. Estela Javier | 433. Ma. Janeth Algas | 674. Sharmaine G. Abalos |
| 193. Ethel Daño | 434. Ma. Jojo Mercado | 675. Shayne Gabriel |
| 194. Evangeline Capul-Sy-Changco | 435. Ma. Karina R. Trinidad | 676. Sheila Mai Rocabo |
| 195. Evelyn A. Siao | 436. Ma. Lourdes S. Imperial, MD | 677. Sherwin Galit |
| 196. Evelyn B. Peñalosa | 437. Ma. Priscilla Jessica Hernandez | 678. Sheryl Santiago-Millares |
| 197. Evelyn G. Sta. Cruz | 438. Ma. Reena Beatrice V. Tagle | 679. Shiela Mae Sarenas |
| 198. Fatima Alcain Dahe | 439. Ma. Rochelle S. Dioquino | 680. SHIELA NAVASCA |
| 199. Fatima Soraya T. Santos | 440. Ma. Rosario Bonagua | 681. Shirley Wong |
| 200. Fay de Ocampo | 441. Ma. Rowela Lamac | 682. Soledad Jalimao |
| 201. Faye Margaret Lagrimas | 442. Ma. Theresa Collante | 683. Stephanie Joy F Ortaleza |
| 202. FE D. ARCENAS | 443. Madelyn Asturias | 684. Stephannie Jude A. Pascual MD |
| 203. Fe Marie Danielle Mendez | 444. Madelynne P. Olalia | 685. STEPHEN JOHN KABALICAN |
| 204. Felice Joy Carlos | 445. Maelady Joan Chua | 686. Susan Diaz |
| 205. Felice Marie Santos | 446. Maida Fatima E. Chan | 687. Terence Jason Flores |
| 206. Felicidad Romualdez | 447. Mar I Jeramil Taguinod | 688. Theodor Vesagas |
| 207. Ferdinand B. Barroga, RMT | 448. Mara Sabrina Clemente | 689. Timothy John S. Bautista, RPh, MD |
| 208. Filipina Ng | 449. Mardy Lee | 690. TOPACIO, ANNA KRISTINA S. |
| 209. Flordeliza Grana | 450. Marfil F. Forteza | 691. Van Aldwin C. Lopez |
| 210. Flory May G. Agustin | 451. Mari Cris Mahinay | 692. Venice Mae T. Mejia |
| 211. Frances Anne Caranto | 452. Maria Alyza I. Ruanto | 693. Venus A. Solar |
| 212. Frances Dianne Ramos | 453. Maria Carmela P. Perez | 694. Veronica Solis |
| 213. Frances Pola Arias | 454. Maria Cecilia Tolentino | 695. Veronica Talla |
| 214. Frances Riel B. Elinzano | 455. Maria Donnabelle U. Dean | 696. Vince Angelo Lim |
| 215. Francesca Michaela Granda | 456. maria frances pinto | 697. Virginia Crisostomo |
| 216. FRANCISCO R. GELLECANAO JR. | 457. Maria Gracia Concepcion C Vesagas | 698. Vivian Joy Abila |
| 217. Frederic Joseph F Diyco, MD | 458. MARIA JOANE R. BONTIA | 699. Vladimir Guillermo |
| 218. GABRIELLE CANTO | 459. Maria Josephine G. Liao | 700. Warren Kemuel M. Pan |
| 219. Gabrielle Dominique Dijamco | 460. Maria Karina M. Montesines | 701. Wedcell Joseph O. Hernandez |
| 220. Gabrielle Paul Pascual | 461. Maria Katrina Manicad | 702. Wendell Adrian Lim |
| 221. Garry A. Montecillo | 462. Maria Korina Abao Dakis | 703. William Lavadia |
| 222. Gemark Montayre | 463. Maria Kristina D. Base | 704. Winston Chan |
| 223. Geneva Beltran | 464. Maria Lilybeth R. Tanchoco | 705. Wren Angelo A. Asprec |
| 224. Genevieve Sadaya, MD | 465. Maria Lourdes Caeg | 706. Wynona Adriano |
| 225. GERALDINE M. ROBLES | 466. Maria Lourdes R. Abeleda | 707. Xandra Mae M. Uy |
| 226. Gerard Raimon M. Saranza | 467. Maria Lourdes T. Solidum | 708. YLA MARLYN LEE A. CORONADO |
| 227. Gerben Villanueva | 468. Maria Margarita Cuesta | 709. Yula Busilan |
| 228. Gian Carlo De Jesus | 469. Maria Niña Grace Bastinen | 710. Zedierick Tanjista |
| 229. gianetta lorena a. gamelo | 470. Maria Rhea F. Sabangan | 711. ZUZETTE B. CATABONA |
| 230. GIANINA DELGADO | 471. Maria Rosario Aguila | 712. RONALD S. GO |
| 231. Ginessa Grace Galang Rendaje | 472. Maria Socorro M. Torno | 713. KARINA MARIE BATU |
| 232. Gingerlie Guerrero | 473. MARIA TERESA BASILIDES | 714. Danwyn Janel A. Mercado |
| 233. Gladdys Christian S. Dela Torre | 474. Maria Teresa C. Luna, MD | 715. Adrian Dwight Martin |
| 234. Glennford Acoba | 475. Maria Teresa Germar | 716. Kento Takahashi |
| 235. GODFREY CACCAM | 476. Maria Teresa T. Rodriguez | 717. Reggiena LP. Lachica |
| 236. Goldie Lynn D. Diaz, MD | 477. Maria Victoria V. Bongar | 718. ROLANDO DE LEON JR |
| 237. Grace Ann Cenon | 478. Marian Almyra S. Naranjilla | 719. Helen Amo |
| 238. Grace Concepcion | 479. Marian Lourdes Ballesteros | 720. Edgardo Lazaro |
| 239. Grace Cuzon | 480. Marica Estrada | 721. Luisa Marie A. Santos |
| 240. GRACE D BACALZO | 481. Maricar Bunyi | 722. SUSANNAH O. SALVADOR, MD, FPDS |
| 241. Grace Diaz | 482. Maricar Valenzuela | 723. Judy Dy Lin-Ong |




